A fuel cell is an electrochemical cell, that produces electricity by converting chemical energy into electrical energy. When hydrogen and oxygen are combined within a fuel cell, heat and electricity are produced, with water vapour produced as a by-product.
Fuel cells have the potential to power electric motors (used within various modes of transportation), provide energy for systems as large as a power station or charge something as small as a mobile phone.
As with battery-electric vehicles (BEV), hydrogen fuel cell electric vehicles (FCEVs), including cars, vans, buses and lorries are powered by electricity, so produce no harmful emissions including carbon dioxide (CO2) from their tailpipe. Only water vapour is produced from hydrogen fuel cell electric vehicles. In FCEVs, energy is stored in the form of compressed hydrogen fuel, rather than in a battery. Hydrogen can be stored and transported at high energy density in liquid or gaseous form.
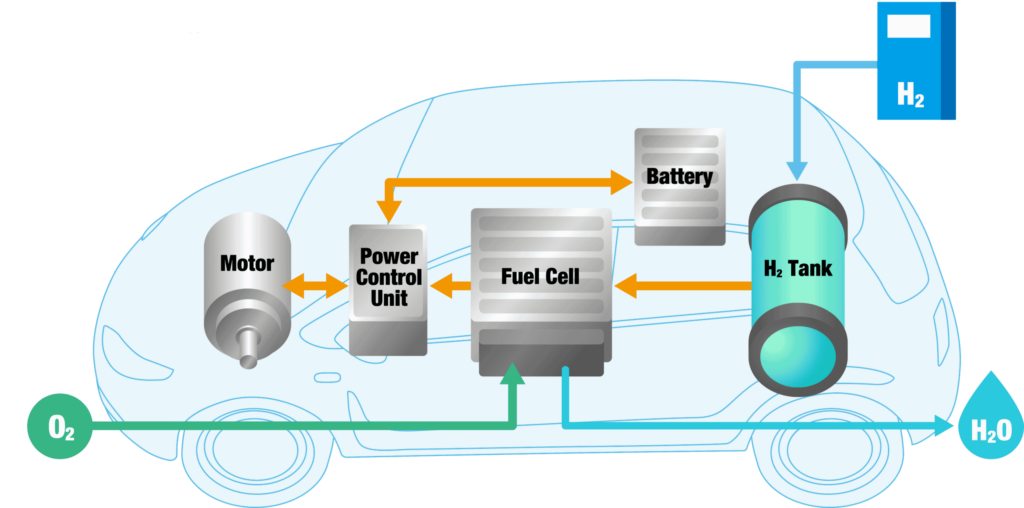
In hydrogen fuel cell electric vehicles (FCEVs) the fuel cell converts compressed hydrogen from their fuel tanks into electricity that powers the electric motor in the vehicle.
A fuel cell coupled with an electric motor is two to three times more efficient than an internal combustion engine running on gasoline. Therefore FCEVs have the advantage of being able to cover longer distances, and only take a few minutes to refuel at a retail site, unlike BEVs that take a long time to recharge in comparison with a much shorter range.
